Below is a titration curve for the titration of 50.0 mL of a 0.100 M solution of the weak acid HA with a 0.100 M solution of NaOH. 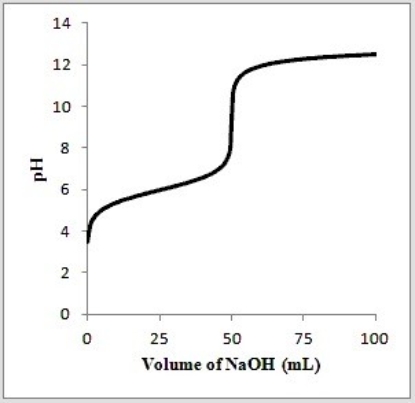 What is the approximate pKa of HA?
What is the approximate pKa of HA?
A) 4
B) 6
C) 8
D) 9
E) 12
Correct Answer:
Verified
Q1: If the pH of a buffer solution
Q92: The amount of strong acid added to
Q93: Which is more soluble in a basic
Q94: Which will precipitate first when AgNO3 is
Q95: Which of the following compounds is appreciably
Q96: Calculate the NaOH concentration necessary to precipitate
Q98: A solution is prepared by mixing 50.0
Q99: Which cation would form an insoluble chloride
Q100: After 50.0 mL of a 0.100 M
Q142: The pH of a solution that is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents