Suppose 3.0 ×10-3 mol of NaOH are added to 6.0 ×10-3 mol of the weak acid HA. Which is the best representation of the final solution at equilibrium? (Each circle represents 1.0 ×10-3 mol of atoms, and the volume of each box is 1.0 L. Solvent water molecules are omitted for clarity.)
A) 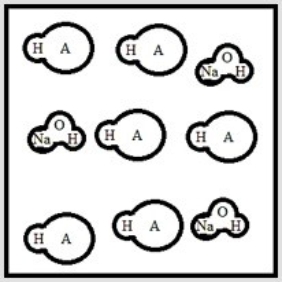
B) 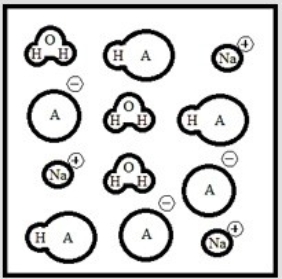
C) 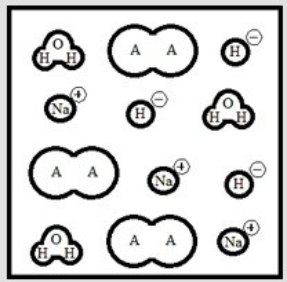
D) 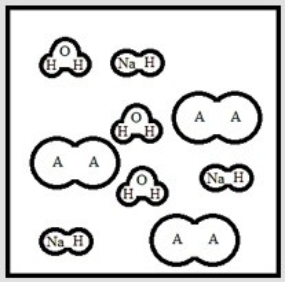
E) 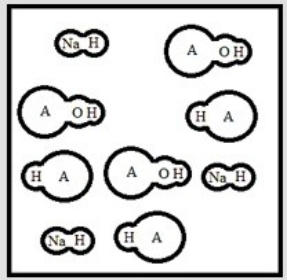
Correct Answer:
Verified
Q76: Calculate the minimum concentration of Cr3+ that
Q77: Calculate the minimum concentration of Mg2+ that
Q79: The solubility of lead(II) chloride is 0.45
Q80: Does a precipitate of magnesium fluoride form
Q82: Suppose 50.0 mL of a 0.100 M
Q83: Suppose 50.0 mL of a 0.100 M
Q84: Which is more soluble in an acidic
Q85: Below is a representation of the sparingly
Q86: Which is more soluble in a basic
Q94: Calculate the solubility of zinc hydroxide, Zn(OH)2,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents