Hydrogen sulfide can be formed in the following reaction: H2(g) + 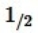 S2(g)
S2(g)  H2S(g) ; ΔH°rxn = -92 kJ/mol
H2S(g) ; ΔH°rxn = -92 kJ/mol
The equilibrium constant,KP,is 106 at 1023 K. Estimate the value of KP at 1218 K. (R = 8.314 J/K • mol)
A) 5.05
B) 18.8
C) 34.7
D) 88.9
E) 598
Correct Answer:
Verified
Q63: Which equation is correct?
A) ΔG = ΔG°
Q64: Calculate KP for the reaction 2NOCl(g)
Q65: Which statement is correct?
A) When Q <
Q66: At 450°C, tert-butyl alcohol decomposes into water
Q67: Iron(III) oxide can be reduced by carbon
Q69: The following reactions occur at 500 K.
Q70: What is the free energy change, ΔG°,
Q71: When the following reaction is at equilibrium,
Q72: If one starts with pure NO2(g) at
Q73: Which statement is correct?
A) If K <
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents