The following reactions occur at 500 K. Arrange them in order of increasing tendency to proceed to completion (least completion → greatest completion) . 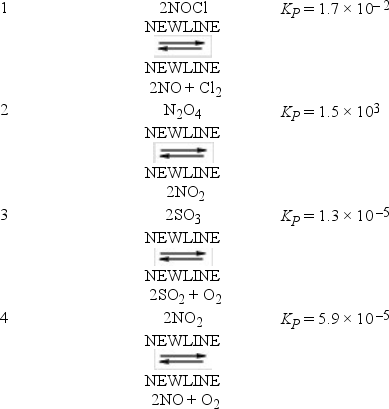
A) 2 < 1 < 3 < 4
B) 3 < 1 < 4 < 2
C) 3 < 4 < 1 < 2
D) 4 < 3 < 2 < 1
E) 4 < 3 < 1 < 2
Correct Answer:
Verified
Q64: Calculate KP for the reaction 2NOCl(g)
Q65: Which statement is correct?
A) When Q <
Q66: At 450°C, tert-butyl alcohol decomposes into water
Q67: Iron(III) oxide can be reduced by carbon
Q68: Hydrogen sulfide can be formed in the
Q70: What is the free energy change, ΔG°,
Q71: When the following reaction is at equilibrium,
Q72: If one starts with pure NO2(g) at
Q73: Which statement is correct?
A) If K <
Q74: Stearic acid, nature's most common fatty acid,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents