For the endothermic reaction A2(g)  2A(g) , a snapshot of an equilibrium mixture of A(g) and A2(g) may look as follows. (Each circle represents 1.0 mol of A atoms, and the volume of the box is 1.0 L.)
2A(g) , a snapshot of an equilibrium mixture of A(g) and A2(g) may look as follows. (Each circle represents 1.0 mol of A atoms, and the volume of the box is 1.0 L.) 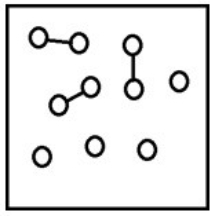 What is the equilibrium constant Kc for this reaction at 298 K? (R = 0.08206 L • atm/K • mol)
What is the equilibrium constant Kc for this reaction at 298 K? (R = 0.08206 L • atm/K • mol)
A) 0.19
B) 0.67
C) 1.3
D) 2.7
E) 5.3
Correct Answer:
Verified
Q66: At equilibrium, the rate of the forward
Q104: For the equilibrium A2(g) Q106: What is the expression for the partial Q107: When the substances in the equation below Q108: When the following reaction is at equilibrium Q110: Solid ammonium hydrogen sulfide is introduced into Q111: For the endothermic reaction A2 (g) Q112: For the endothermic reaction A2(g) Q113: Which of the following statements is incorrect Q114: When the reaction 2H2S(g) ![]()
2NOCl(g)
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents