Consider reactions A, B, and C, which have the potential energy profiles shown. Assuming that the reactions have roughly the same frequency factors, which reaction is the slowest? 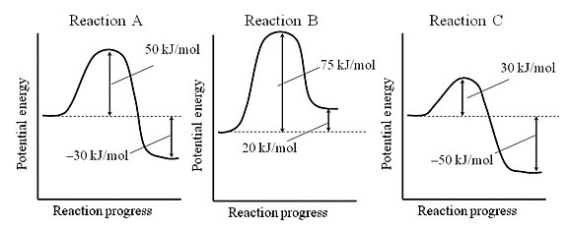
A) Reaction A
B) Reaction B
C) Reaction C
D) All of the reactions have the same rate.
Correct Answer:
Verified
Q76: What is the integrated rate law for
Q77: The radioactive isotope tritium decays with a
Q78: A reactant R is being consumed in
Q79: What rate constant can be determined from
Q80: What is the integrated rate law for
Q82: Enzymes are _ .
A) large carbohydrate molecules
B)
Q83: A rate constant obeys the Arrhenius equation,
Q84: Consider the following potential energy profile for
Q85: What is the name given to the
Q86: Consider the following potential energy profile for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents