Which is true concerning the following potential energy diagram? 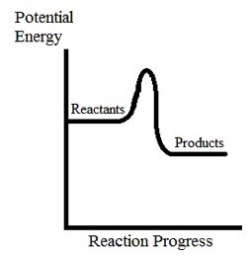
A) This reaction is exothermic and has a positive activation energy.
B) This reaction is endothermic and has a positive activation energy.
C) This reaction is exothermic and has a negative activation energy.
D) This reaction is endothermic and has a negative activation energy.
E) This reaction is thermoneutral and has a zero activation energy.
Correct Answer:
Verified
Q90: The following is an Arrhenius plot of
Q91: The following diagram represents the first-order decomposition
Q92: The following diagram represents the first-order decomposition
Q93: The following diagram represents the zeroth-order decomposition
Q94: The activation energy for the following first-order
Q96: The isomerization of cyclopropane follows first-order kinetics.
Q97: Consider the following potential energy profile for
Q98: What is the molecularity of the following
Q99: Consider the following potential energy profile for
Q100: The isomerization of methyl isocyanide, CH3NC →
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents