The following is an Arrhenius plot of a first-order reaction. The rate constant is measured in units of s-1. 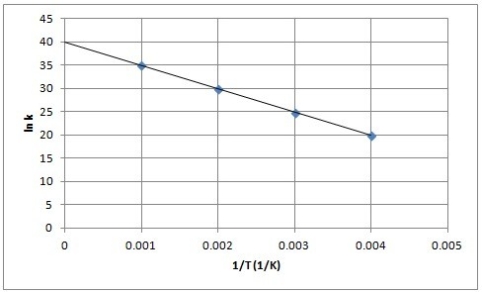 Based on this Arrhenius plot, what is the Arrhenius frequency factor (A) of the reaction? (R = 8.314 J/K mol)
Based on this Arrhenius plot, what is the Arrhenius frequency factor (A) of the reaction? (R = 8.314 J/K mol)
A) 5.0 s-1
B) 40 s-1
C) 50 s-1
D) 5.0 × 103 s-1
E) 2.4 × 1017 s-1
Correct Answer:
Verified
Q1: A transition state is a species (or
Q17: The rate of a reaction is determined
Q20: The rate law cannot be predicted from
Q102: A _-_ is a reaction whose rate
Q103: A(n) _ increases the reaction rate without
Q104: The following is an Arrhenius plot of
Q107: The intermediate in a reaction appears in
Q108: _ describes the reaction rate as being
Q109: The _ is the equation relating the
Q111: A(n) _ is the name given to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents