Which figure best illustrates the hybrid orbitals on carbon in benzene, C6H6?
A) 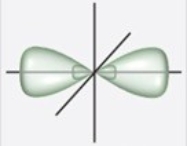
B) 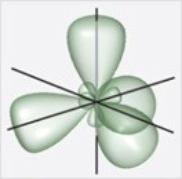
C) 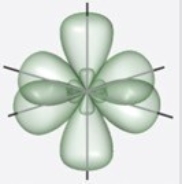
D) 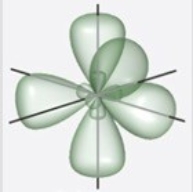
E) 
Correct Answer:
Verified
Q79: A molecule with the formula AB4 and
Q80: How many bonds are there in one
Q81: Which figure best illustrates the hybrid orbitals
Q82: Consider the species F2+, F2, and F2-.
Q83: For a polyatomic ion having the general
Q85: The nitrosonium ion, NO+, forms a number
Q86: Consider the species N2-, N2, and N2+.
Q87: According to molecular orbital theory, what is
Q88: For a homonuclear diatomic molecule, which molecular
Q89: Which molecular formula corresponds to a structural
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents