Aluminum oxide can be reduced to aluminum metal using carbon, the other reaction product being carbon monoxide. What is the enthalpy change if 12.50 g of aluminum is produced by this method? 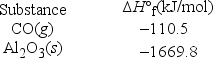
A) 310.0 kJ
B) 386.8 kJ
C) 412.4 kJ
D) 773.6 kJ
E) 824.8 kJ
Correct Answer:
Verified
Q65: Which of the following processes always results
Q94: How much heat is released if 35.0
Q95: Ozone (O3) in the atmosphere can be
Q96: How much heat is released if 7.15
Q97: What is ΔH°rxn for the following reaction?
Q98: An important step in the synthesis of
Q100: Solid sodium peroxide (Na2O2) reacts with liquid
Q101: The heat absorbed by a system at
Q102: For which of the substances below is
Q108: A home aquarium is an example of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents