Ethanol, C2H5OH, is promoted as a clean fuel and is used as an additive in many gasoline mixtures. Calculate the ΔH°rxn for the combustion of ethanol. 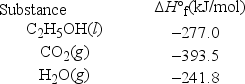
A) -1235.4 kJ
B) -751.8 kJ
C) -358.3 kJ
D) 358.3 kJ
E) 1235.4 kJ
Correct Answer:
Verified
Q77: What is the standard enthalpy change for
Q78: What is ΔH°rxn for the following reaction?
Q79: Given that CaO(s) + H2O(l) → Ca(OH)2(s),
Q80: Which equation has a ΔHrxn that is
Q81: What is ΔH°rxn for the decomposition of
Q83: Sand is converted to pure silicon in
Q84: How much heat is evolved if 0.600
Q85: Pentaborane B5H9(s) burns vigorously in O2 to
Q86: What is ΔH°rxn for the following reaction?
Q87: What is ΔH°rxn for the following reaction?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents