Using the table below, what is the best method for obtaining energy from catabolizing ethanol, and why? A.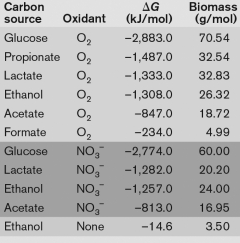
A) the catabolism of ethanol without an oxidant, because the G is almost positive
B) the oxidation of ethanol with as the oxidant is because of the large yield of energy released
C) the oxidation of ethanol with oxygen, because the G is the largest negative number indicating the large yield of energy released.
D) The oxidation of ethanol yields too little biomass to be catabolized.
E) None-ethanol can only be catabolized syntrophically.
Correct Answer:
Verified
Q1: Glycolytic reactions with a near-zero
Q2: A metabolic process allowing for anaerobic
Q4: The molecule shown below carries energy in
Q5: Hydrogen-generating reactions in Syntrophus and Syntrophomonas
Q6: In many bacteria, the electron carrier _
Q7: In most environments, the nutrient concentrations outside
Q8: The laws of thermodynamics indicate that systems
Q9: In amphibolic pathways, enzymes do NOT
A) function
Q10: Which of the following statements is NOT
Q11: Which of the following phenomena is sufficient
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents