
Use the following figure to answer the question.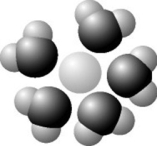
Based on your knowledge of the polarity of water molecules, the solute molecule depicted is most likely ________.
A) positively charged
B) negatively charged
C) without charge
D) nonpolar
Correct Answer:
Verified
Q4: One mole (mol) of glucose (molecular mass
Q10: Which of the following effects can occur
Q11: Which of the following can be attributed
Q16: Melting of ice and thus reduced feeding
Q19: Liquid water _.
A) is less dense than
Q23: The molar mass of glucose is 180
Q25: Which of the following statements is true
Q26: One of the buffers that contribute
Q27: A solution contains 0.0000001 (10⁻⁷) moles of
Q30: When an ionic compound such as sodium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents