
Use the following figure to answer the question.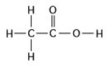
Two moles of the compound in the figure would weigh how many grams? (Note: The atomic masses, in daltons, are approximately 12 for carbon, 1 for hydrogen, and 16 for oxygen.)
A) 30
B) 60
C) 90
D) 120
Correct Answer:
Verified
Q21: Carbon dioxide in the atmosphere dissolves with
Q26: One of the buffers that contribute
Q26: What is the pH of a solution
Q27: Identical heat lamps are arranged to shine
Q27: A solution contains 0.0000001 (10⁻⁷) moles of
Q28: Consider the following reaction at equilibrium:
Q30: Which of the following is considered to
Q32: A 0.01 M solution of a substance
Q33: How much of 0.5 M glucose (molecular
Q39: How does 0.5 M sucrose (molecular mass
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents