Alkaloid salts are not very soluble in the organic solvent diethyl ether. What might happen to the free-base form of caffeine dissolved in diethyl ether if gaseous hydrogen chloride, HCl, were bubbled into the solution? 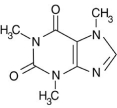
A) A second layer of water would form.
B) Nothing, and the HCl gas would merely bubble out of solution.
C) The diethyl ether insoluble caffeine salt would form as a white precipitate.
D) The acid/base reaction would release heat, which would cause the diethyl ether to start evaporating.
Correct Answer:
Verified
Q63: Which of the following commonly acts as
Q68: The phosphoric acid salt of caffeine has
Q69: Q69: If you saw the label "phenylephrine HCl" Q71: An amine can often form R-NH₃+ (where Q72: In water, does the molecule lysergic acid Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()

