Given the following diagram, describe what happens electronically between these two molecules. 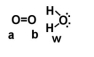
A) Oxygen A becomes slightly positively charged due to the protons on the water molecule.
B) Oxygen B becomes slightly positively charged due to the protons on the water molecule.
C) Oxygen A becomes slightly negatively charged due to the oxygen molecule.
D) Hydrogens on oxygen W becomes slightly positively charged due to the oxygen molecule.
E) none of the above
Correct Answer:
Verified
Q110: What is happening at the molecular level
Q115: Which of the following describes an aqueous
Q122: What is a hydrogen bond?
A)a special type
Q127: Given the above diagram, describe what happens
Q129: What is the difference between a dipole-dipole
Q131: Which of the following is most likely
Q134: Which of the following is the main
Q135: Which of the following would have the
Q138: Which of the following intermolecular forces best
Q139: Which of the following would have the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents