Account for the observation that ethyl alcohol, C2H5OH, dissolves readily in water but dimethyl ether, CH3OCH3, which has the same number and kinds of atoms, does not. 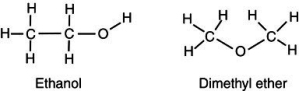
A) The hydrogens on the dimethyl ether surround the molecule, shielding the inner atoms from interacting with the water.
B) Because the carbons arrange themselves in a straight line, the ethanol can interact more easily with more water molecules, thus increasing its solubility.
C) The high electronegativity of the carbon-oxygen-carbon bond on dimethyl ether creates a strong dipole charge on the ends of the molecule, making it highly soluble in water.
D) Because dimethyl ether lacks an -OH group, it is significantly less polar than is ethyl alcohol and is not readily soluble in water.
Correct Answer:
Verified
Q65: What property primarily determines the effect of
Q74: If you were to increase the pressure
Q82: Which solute graphed above has a solubility
Q91: Suggest why sodium chloride,NaCl,is insoluble in gasoline.Consider
Q95: When you set a pot of tap
Q241: The ionic bonds within cesium chloride, CsCl,
Q243: Would you expect a hydrogen atom with
Q245: Why are dipole-dipole molecular interactions stronger than
Q246: A molecular interaction not discussed in Chapter
Q247: How are the ionic and covalent bonds
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents