Suppose that a certain atom possesses only four distinct energy levels. Assuming that all transitions between levels are possible, how many spectral lines will this atom exhibit? 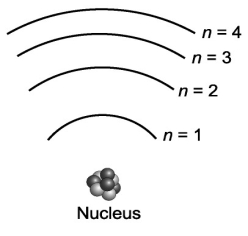
A) three
B) four
C) six
D) unlimited
Correct Answer:
Verified
Q100: Which of the following statements is true
Q105: What property of an electron makes it
Q106: How is the term photon related to
Q110: What is the main tenet of Plank's
Q111: What is the relationship between the light
Q113: Which color of light comes from the
Q118: Light is emitted as an electron transitions
Q119: Which of the following statements about electrons
Q121: Which would produce a more highly valued
Q133: How is it possible to deduce the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents