Consider the various frequencies of the three photons emitted from the following three individual electron transitions in the figure below: n=3 to n=2; n=2 to n=1; n=3 to n=1. These transitions would produce three spectral lines in a spectroscope. If the energy spacing between the levels were equal, would this affect the number of spectral lines? 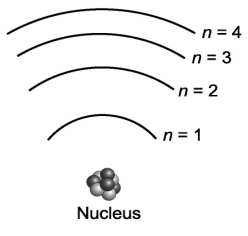
A) Yes, two otherwise separate lines would converge into a single more intense line.
B) No, but the spacing between the spectral lines would change.
C) Yes, two otherwise separate lines would converge into a single less intense line.
D) No, but some would then require a prism in order to be seen.
Correct Answer:
Verified
Q15: What is the main difference between a
Q83: Which of the following could be represented
Q104: How can a hydrogen atom,which has only
Q112: How might the spectrum of an atom
Q114: What was Niels Bohr's explanation for the
Q116: Which of the following best explains what
Q126: In some instances electromagnetic radiation behaves like
Q128: How does the wave model of electrons
Q131: An atom absorbs or emits only particular
Q137: Some older cars vibrate loudly when driving
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents