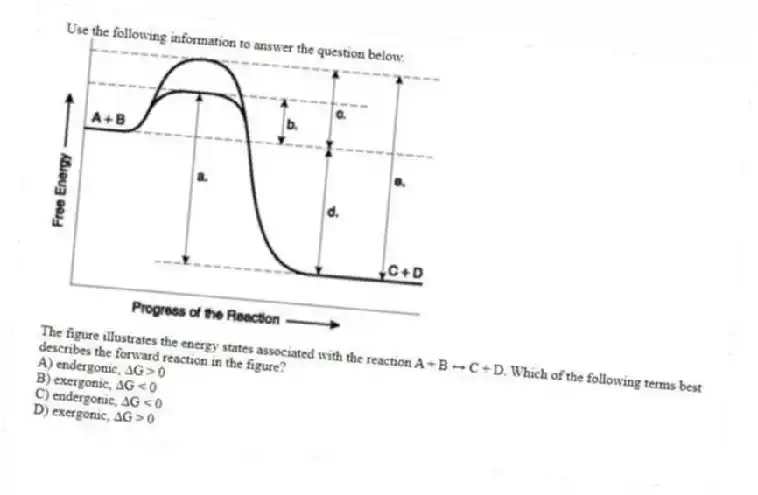
Use the following information to answer the question below.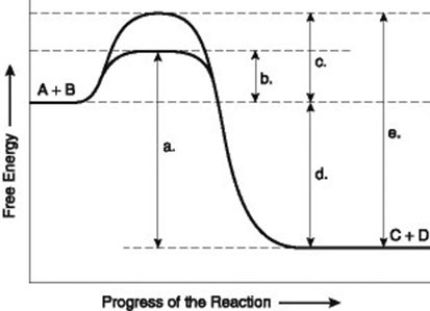
The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following terms best describes the forward reaction in the figure?
A) endergonic, ∆G > 0
B) exergonic, ∆G < 0
C) endergonic, ∆G < 0
D) exergonic, ∆G > 0
Correct Answer:
Verified
Q49: HIV is the virus that causes AIDS.
Q51: In a metabolic pathway, succinate dehydrogenase catalyzes
Q52: Q53: Use the following information to answer the Q53: In addition to activating or inhibiting enzymes Q55: Use the following information to answer Q55: A series of enzymes catalyze the reactions Q56: How might a change of one amino Q57: Use the following information to answer the Q58: Which of the following graphs most likely![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents