The isotope of carbon commonly referred to as "carbon-14" is
A) 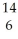 C.
C.
B) 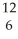 C.
C.
C) 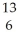 C.
C.
D) 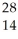 Si.
Si.
Correct Answer:
Verified
Q42: The experiment that confirmed the existence of
Q45: The proton has
A)the same mass and charge
Q46: Which contains 7 neutrons? Q48: The neutron has Q50: How many nucleons are in this isotope Q50: The mass of a nucleon is Q51: The charge on the nucleus of a Q53: The element iron (Fe)occurs naturally as four Q59: The sum of protons and neutrons in Q60: How many nucleons are there in the
A) ![]()
A)the same approximate mass and
A)1 gram.
B)1
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents