Given the following Ksp values, determine which solution has the smallest concentration of ions at equilibrium. 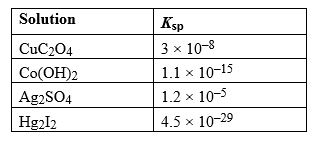
A) CuC2O4
B) Co(OH) 2
C) Ag2SO4
D) Hg2I2
Correct Answer:
Verified
Q10: All the reactants are completely changed to
Q11: Which conditions are present when K is
Q12: Is the LD50 a measurement for acute
Q13: Which of the following describes how a
Q14: Determine the solubility equilibrium constant for CaF2
Q15: Which statement is true for a reaction
Q16: Which statement describes when dynamic equilibrium occurs?
A)
Q18: Given the following list of Ka values,
Q19: Carbon monoxide poisoning occurs when hemoglobin binds
Q20: The equilibrium constant for a reaction is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents