Given the following list of Ka values, determine which weak acid solution would have the lowest pH value at equilibrium assuming all acids have the same initial concentration. 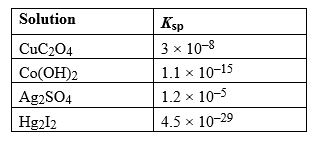
A) HIO3
B) H2C6H6O6
C) H2O2
D) H3AsO4
Correct Answer:
Verified
Q1: Which classification contains substances that decompose the
Q2: Which of the following refers to a
Q3: How does the presence of a catalyst
Q5: Determine the equilibrium concentration of bromide when
Q6: Which of the following refers to a
Q7: Determine the equilibrium concentration of cadmium when
Q8: Which of the following describes how a
Q9: Given the following Ksp values, determine which
Q10: All the reactants are completely changed to
Q11: Which conditions are present when K is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents