A water molecule, as shown here, is polar because of 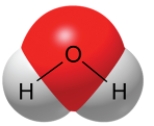
A) the transfer of electrons.
B) unequal sharing of electrons.
C) its ability to freeze.
D) its hydrogen bonds.
E) the negative charge of the molecule.
Correct Answer:
Verified
Q8: Which of the following properties of water
Q9: When a sodium atom transfers an electron
Q10: Positron-emission tomography (PET) is an example of
Q11: The type of bond that would form
Q12: Glucose, C 6H 12O 6, is best
Q14: Which of the following is a molecule?
A)
Q15: Which of the following is a way
Q16: The figure below is depicting the interaction
Q17: When a positively charged hydrogen in a
Q18: Which of the following statements about atoms
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents