Which of the following laws states 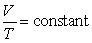 for a given amount of a gas, when pressure is constant?
for a given amount of a gas, when pressure is constant?
A) Charles's law
B) Boyle's law
C) Avogadro's law
D) Graham's law
E) Gay-Lussac's law
Correct Answer:
Verified
Q18: The equation, PV = nRT is called
Q19: According to the kinetic theory of gases,
Q20: The combined gas law considers the amount
Q21: A gas has an initial pressure of
Q22: What is the Kelvin equivalent of 145°C?
A)
Q24: is a gas that deviates slightly from
Q25: Pressure at a point deep in an
Q26: Boyle's law explains the relationship between _.
A)
Q27: The vapor pressure of water increases with
Q28: Which of the following statements is true
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents