True/False
Given: 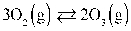 If the Keq at 57.0°C for the reaction above is 13.6, then KP is 0.0474.
If the Keq at 57.0°C for the reaction above is 13.6, then KP is 0.0474.
Assume R = 0.08205 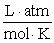
Correct Answer:
Verified
Related Questions
Q8: Given: Q9: A catalyst does not affect the extent Q10: In a chemical equilibrium reaction all the Q11: Given: Q12: Chemical equilibrium is static rather than dynamic. Q14: Given: Q15: The Ka for HClO2 is 1.10 × Q16: An equilibrium equation should be balanced. Q17: The equilibrium concentration of H2O(g) for the Q18: Given: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()

