What is the KP at 37.0°C for this reaction if the Keq is 1.20 × 10−2? 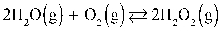 Assume R = 0.08205
Assume R = 0.08205 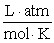
A) 4.27 * 10- 4
B) 2.47 *10- 4
C) 2.74 * 10- 4
D) 7.42 * 10- 4
E) 4.72 *10- 4
Correct Answer:
Verified
Q22: If the equilibrium partial pressures of [PCl5],
Q23: If [C2H2], [O2], [H2O], and [CO2] are
Q24: What is the Keq expression for the
Q25: What is the Keq expression for the
Q26: What is the Keq expression for the
Q28: What is the Keq expression for the
Q29: If [H2], [Br2], and [HBr] are 0.0350
Q30: The equilibrium partial pressures of [H2O2] and
Q31: If [C2H6], [O2], [CO2], and Keq are
Q32: If the Kb for CN− is 1.6
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents