What is the correct KP expression for this reaction? 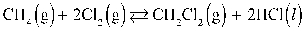
A) 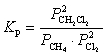
B) 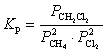
C) 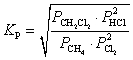
D) 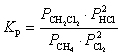
E) 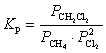
Refer to the reaction below for 46-49.
Given: 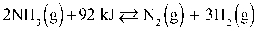
Correct Answer:
Verified
Q54: Determine the equilibrium concentration of CO(g) for
Q55: To maximize the amount of CO2 the:
A)
Q56: Determine the equilibrium concentration of N2O3(g) for
Q57: What is the correct Keq expression for
Q58: Determine the equilibrium concentration of Cl2(g) for
Q60: Which of the following will cause the
Q61: What is the concentration of [Ag+] in
Q62: Which of the following is the equilibrium
Q63: What is the pOH of a 0.500
Q64: Describe chemical equilibrium as a dynamic process.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents