To maximize the amount of N2 the:
A) pressure of the reaction should be reduced.
B) temperature of the reaction should be decreased.
C) amount of catalyst added should be increased.
D) number of moles of N2 in the reaction should be increased.
E) number of moles of NH3 in the reaction should be reduced.
Refer to the reaction below for 50-53.
Given: 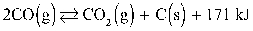
Correct Answer:
Verified
Q47: Determine the equilibrium concentration of HCN(g) for
Q48: To maximize the amount of CO the:
A)
Q49: If concentration is defined as x, formulate
Q50: Determine the equilibrium concentration of H2(g) for
Q51: Which of the following will cause the
Q53: To maximize the amount of NH3 the:
A)
Q54: Determine the equilibrium concentration of CO(g) for
Q55: To maximize the amount of CO2 the:
A)
Q56: Determine the equilibrium concentration of N2O3(g) for
Q57: What is the correct Keq expression for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents