What is the work done on the gas as it expands from pressure P1 and volume V1 to pressure P2 and volume V2 along the indicated straight line? 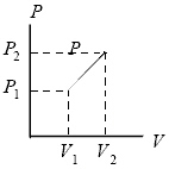
A) (P1 + P2) (V1 - V2) /2
B) (P1 + P2) (V1 - V2)
C) (P1 - P2) (V1 + V2)
Correct Answer:
Verified
Q3: According to the first law of thermodynamics,the
Q5: Which of the following increases the internal
Q6: An adiabatic expansion refers to the fact
Q11: A 4-mol ideal gas system undergoes an
Q12: During an isobaric process which one of
Q12: A closed 2.0-L container holds 3.0 mol
Q14: If an ideal gas does positive work
Q15: A 5-mol ideal gas system undergoes an
Q16: Area on a PV diagram has units
Q17: A 2.0-mol ideal gas system is maintained
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents