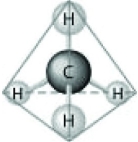
 Which of the following factors contribute to the tetrahedral shape of the above molecule?
Which of the following factors contribute to the tetrahedral shape of the above molecule?
A) the shape of the two p orbitals in the carbon atom
B) the shape of the one s orbital in the carbon atom
C) the shape of the sp3 hybrid orbitals of the electrons shared between the carbon and hydrogen atoms
D) hydrogen bonding configurations between the carbon and hydrogen atoms
Correct Answer:
Verified
Q38: Based on electron configuration, which of the
Q39: How many electrons are present in a
Q40: Oxygen has an atomic number of 8
Q41: How many electrons participate in a triple
Q42: Which of the following statements correctly describes
Q44: An atom has four electrons in its
Q45: The atomic number of sulfur is 16.
Q46: Which one of the following describes the
Q47: Which of the following factors will increase
Q48: If an atom has a charge of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents