Figure 7-1
Use the figure to answer the corresponding question(s) . 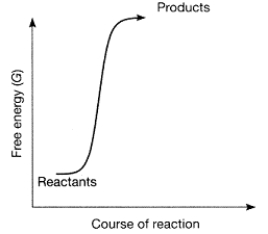
-Which of the following statements about Figure 7-1 is true?
A) The reactants have more free energy than the products.
B) The products have more free energy than the reactants.
C) The figure represents a spontaneous reaction.
D) The figure represents an endergonic reaction.
E) The reaction is endergonic, and also the products have more free energy than the reactants.
Correct Answer:
Verified
Q34: Select the hydrogen acceptor molecule that stores
Q35: The reaction ATP + H2O
Q36: Figure 7-2
Use the figure to answer the
Q37: _ is a process where energy (as
Q38: Because enzymes affect the speed of chemical
Q40: Consider the following two chemical equations:
Q41: If one continues to increase the temperature
Q42: Figure 7-2
Use the figure to answer the
Q44: Hydrolases are one important class of enzyme
Q56: A closed system does not exchange energy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents