The two molecules in the following figure represent: 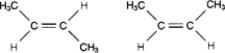
A) ionized structures.
B) enantiomers.
C) secondary structures.
D) geometric isomers.
E) polymers.
Correct Answer:
Verified
Q2: The highly polarized nature of compounds containing
Q3: Figure 3-1
Use the figure below to answer
Q4: Which of the following is not a
Q5: Which pair is mismatched?
A) monsaccharide-maltose
B) disaccharide-sucrose
C) polysaccharide-cellulose
D)
Q6: Hydrocarbons are hydrophobic because:
A) the covalent bonds
Q7: Amyloplasts are organelles that store:
A) fat.
B) starch.
C)
Q8: Carbohydrate molecules:
A) serve as structural components of
Q9: The difference between a hexose and a
Q10: A chemical reaction in which polymers are
Q11: The chemical interactions of large organic molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents