When solutions of NaCl and 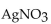 are mixed,
are mixed,
A) a precipitate of AgCl forms.
B) precipitates of 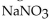 and AgCl form.
and AgCl form.
C) a precipitate of  forms.
forms.
D) no precipitate forms.
E) a precipitate of 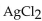 forms.
forms.
Correct Answer:
Verified
Q19: Q20: An increase in the temperature of a Q21: When solutions of Q22: Which one of the following compounds will Q23: Rubbing alcohol is 70.% (v/v)isopropyl alcohol by Q25: Which one of the following compounds will Q26: Which one of the following compounds will Q27: How many milliliters of a 25% Q28: What volume (mL) of a Q29: What is the molarity of a solution![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents