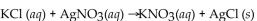 How many mL of 0.234 M KCl solution will react completely with 25.0 mL of 0.168 M
How many mL of 0.234 M KCl solution will react completely with 25.0 mL of 0.168 M 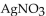 solution?
solution?
A) 35.6 mL
B) 17.9 mL
C) 34.8 mL
D) 25.0 mL
E) 22.5 mL
Correct Answer:
Verified
Q35: The mass/volume percent concentration refers to
A)grams of
Q36: How many grams of glucose are needed
Q37: What is the molarity of a solution
Q38: According to Henry's law, the solubility of
Q39: Which one of the following compounds will
Q41: What is the molarity of a solution
Q42: What is the molarity of a KCl
Q43: How many moles of Q44: What is the final mass/volume (m/v)% of Q45: What is the molarity of a solution![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents