0.50 mole of 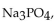 a strong electrolyte, is added to 1.0 kg of water. The freezing point of the solution will be
a strong electrolyte, is added to 1.0 kg of water. The freezing point of the solution will be
A) 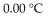
B) 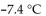
C) 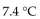
D) 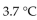
E) 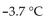
Correct Answer:
Verified
Q43: A substance that produces only a small
Q49: A substance that carries an electric current
Q70: A solution with the same osmotic pressure
Q71: A red blood cell will undergo hemolysis
Q72: 1.0 mole of NaCl, a strong electrolyte,
Q73: Methanol, CH3OH, can be classified as a
Q74: The process by which a semipermeable membrane
Q77: The molarity of a solution of 5.0
Q78: A mixture in which one component settles
Q79: If 100. mL of water is added
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents