A gas at 5.00 atm pressure was stored in a tank during the winter at 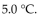 During the summer, the temperature in the storage area reached
During the summer, the temperature in the storage area reached 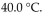 What was the pressure in the gas tank then, if volume and amount of gas do not change?
What was the pressure in the gas tank then, if volume and amount of gas do not change?
A) 69.5 atm
B) 5.63 atm
C) 40.0 atm
D) 0.625 atm
E) 4.44 atm
Correct Answer:
Verified
Q19: Which measurement describes the pressure of a
Q20: According to Boyle's Law, the pressure of
Q21: Complete the following statement: According to Charles's
Q22: Vapor pressure can be described as
A)the pressure
Q23: A balloon is filled with helium gas.
Q25: A gas contained in a steel tank
Q26: An autoclave is used to sterilize surgical
Q27: A gas sample in a closed, expandable
Q28: According to Gay-Lussac's Law, the pressure of
Q29: When the combined gas law is rearranged
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents