A sample of nitrogen gas had a volume of 500 .mL, a pressure in its closed container of 740 torr, and a temperature of 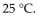 What was the new volume of the gas when the temperature was changed to
What was the new volume of the gas when the temperature was changed to 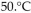 and the new pressure was 760 torr, if the amount of gas does not change?
and the new pressure was 760 torr, if the amount of gas does not change?
A) 400 mL
B) 240 mL
C) 450 mL
D) 970 mL
E) 530 mL
Correct Answer:
Verified
Q37: As the temperature of a gas increases,
Q38: A diver exhales a bubble with a
Q39: What unit of temperature is used in
Q40: The boiling point of water at sea
Q41: According to Avogadro's law,
A)the volume of a
Q43: The total pressure in a mixture of
Q44: Which of the following is NOT a
Q45: At STP, what is the volume of
Q46: How many moles of neon occupy a
Q47: At STP, how many moles of helium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents