A tank contains helium gas at 490 mmHg , nitrogen gas at 0.75 atm, and neon at 520 torr. What is the total pressure in atm?
A) 0.55 atm
B) 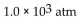
C) 1.5 atm
D) 2.1 atm
E) 1600 atm
Correct Answer:
Verified
Q59: At STP, what is the volume of
Q60: A cyclopropane-oxygen mixture is used as an
Q61: Boyle's law is usually written as _.
Q62: In the kinetic molecular theory of gas
Q63: A gas sample contains 4.0 g of
Q65: A barometer is a device for measuring
Q66: The relationship Q67: The relationship Q68: If atmospheric pressure on a certain day Q69: Oxygen makes up about _ percent of![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents