In a gas mixture, the partial pressures are:  76.0 torr, He 230 mmHg , and
76.0 torr, He 230 mmHg , and 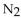 0.640 atm. The total pressure in mmHg is
0.640 atm. The total pressure in mmHg is
A) 792 mmHg
B) 306 mmHg
C) 760 mmHg
D) 562 mmHg
E) 486 mmHg
Correct Answer:
Verified
Q42: A barometer is usually filled with _.
Q55: The pressure unit 1 mmHg is the
Q60: Nitrogen makes up about _ percent of
Q71: Gay-Lussac's law is usually written as _.
Q72: The pressure exerted by the particles of
Q73: One atmosphere is the same as _
Q74: A tank contains a mixture of helium,
Q77: Which of the following correctly describes the
Q78: Charles's law is usually written as _.
Q79: The relationship ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents