What is the molar mass of sodium phosphate, 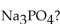
A) 308 g
B) 119 g
C) 164 g
D) 226 g
E) 354 g
Correct Answer:
Verified
Q28: Calculate the molar mass of potassium chloride,
Q29: How many moles of carbon are there
Q30: What is the molar mass of copper(II)
Q31: In this reaction, what is the substance
Q32: The molar mass of C3H8O2 is
A)52.0 g.
B)69.0
Q34: How many moles of iron are present
Q35: What is oxidized and what is reduced,
Q36: One mole of neon has a mass
Q37: One mole of particles of any substance
Q38: In an oxidation-reduction reaction, the substance reduced
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents