In the reaction of nitrogen gas, 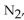 with hydrogen gas,
with hydrogen gas, 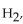 to form ammonia gas,
to form ammonia gas, 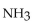 , how many moles of hydrogen are needed to react with two moles of nitrogen?
, how many moles of hydrogen are needed to react with two moles of nitrogen?
A) 6 moles
B) 8 moles
C) 4 moles
D) 10 moles
E) 2 moles
Correct Answer:
Verified
Q38: In an oxidation-reduction reaction, the substance reduced
Q39: In an oxidation-reduction reaction, the substance oxidized
Q40: The molar mass of potassium is
A)
Q41: How many grams of glucose 
Q42: 4.00 moles of sodium have a mass
Q45: For the question(s)that follow, consider the following
Q46: When 4 moles of aluminum are allowed
Q47: For the question(s)that follow, consider the following
Q48: 0.100 mole of lithium has a mass
Q53: For the following question(s),consider the following equation.
2Mg
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents