In the reaction of silver nitrate with sodium chloride, how many grams of silver chloride will be produced from 100. g of silver nitrate when it is mixed with an excess of sodium chloride? The equation for the reaction is below. 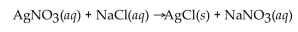
A) 107.9 g
B) 169.9 g
C) 58.9 g
D) 84.4 g
E) 0.589 g
Correct Answer:
Verified
Q75: Q76: When 85.0 g of Q77: When 85.0 g of Q78: How many grams of hydrogen are needed Q79: Any reaction that absorbs 150 kcal of Q81: What type of reaction is the following? Q82: What type of reaction is the following? Q83: What is the molar mass of NaBr? Q84: Barium chloride and sodium sulfate react according Q85: What is the molar mass of helium?![]()


Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents