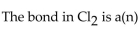
A) metallic bond.
B) nonpolar covalent bond.
C) ionic bond.
D) polar ionic bond.
E) no bond.
Correct Answer:
Verified
Q71: Which of the following compounds contains an
Q72: The shape of the ammonia molecule
Q73: The water molecule has a dipole with
Q74: The strongest interactions between molecules of hydrogen,
Q75: The shape of the carbon dioxide molecule
Q77: A polar covalent bond is found in
Q78: If the electronegativity difference between elements X
Q79: The shape of the carbon tetrachloride molecule
Q80: The carbon tetrachloride molecule, Q81: The strongest interactions between molecules of iodine,![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents