A 10.0 mL of 0.121 M 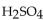 is neutralized by 17.1 mL of KOH solution. The molarity of the KOH solution is
is neutralized by 17.1 mL of KOH solution. The molarity of the KOH solution is
A) 0.142 M
B) 0.428 M
C) 0.207 M
D) 0.0708 M
E) 0.4141 M
Correct Answer:
Verified
Q41: How many moles of Q42: What is the molarity of a KOH Q43: A 25.0 mL sample of Q44: In a buffer system of HF and Q45: In a neutralization reaction, how many moles Q47: The neutralization reaction between Q48: Q49: The function of a buffer is to Q50: Which of the following is a buffer Q51: The normal blood pH is about![]()
![]()

A)maintain
A)7.00.
B)7.20.
C)7.40.
D)6.80.
E)7.60.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents