The equilibrium constant for the reaction for the decomposition of 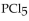 to chlorine and
to chlorine and 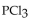 at a certain temperature is 0.042 .
at a certain temperature is 0.042 .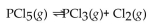
If the equilibrium concentrations are 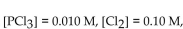 what is the value of
what is the value of 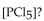
A) 0.0010 M
B) 0.042 M
C) 0.0020 M
D) 0.010 M
E) 0.024 M
Correct Answer:
Verified
Q14: What is the correct form of the
Q15: A reaction that can proceed in either
Q16: The equation for the formation of ammonia
Q17: In a catalyzed chemical reaction, one function
Q18: What is the correct form of the
Q20: For the following reaction, the equilibrium concentrations
Q21: The reaction of hemoglobin with oxygen can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents