In the following gas phase reaction, Kc is much less than 1. At equilibrium, which of the following statements is true? 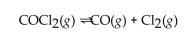
A) The concentration of products is much greater than the concentration of reactants.
B) A catalyst will increase the concentration of products formed.
C) The concentration of reactant is much greater than the concentration of products.
D) The concentrations of products and reactants are approximately equal.
Correct Answer:
Verified
Q5: The reaction for the decomposition of
Q6: In any chemical reaction, the rate of
Q7: The equilibrium constant for the production of
Q8: Which of the following equilibrium constants indicates
Q9: When a reaction is at equilibrium,
A)the products
Q11: Refrigerating perishable foods affects biochemical reactions by
A)improving
Q12: The activation energy of a chemical reaction
Q13: The value of the equilibrium constant for
Q14: What is the correct form of the
Q15: A reaction that can proceed in either
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents