The equilibrium constant for the production of carbon dioxide from carbon monoxide and oxygen is 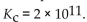
This means that the reaction mixture at equilibrium is likely to consist of
A) mostly starting materials.
B) twice as much product as starting material.
C) an equal mixture of products and reactants.
D) mostly products.
E) twice as much starting material as product.
Correct Answer:
Verified
Q2: A catalyst is
A)a reactant in a chemical
Q3: A chemical reaction has reached equilibrium when
A)all
Q4: The rate of any chemical reaction can
Q5: The reaction for the decomposition of
Q6: In any chemical reaction, the rate of
Q8: Which of the following equilibrium constants indicates
Q9: When a reaction is at equilibrium,
A)the products
Q10: In the following gas phase reaction, Kc
Q11: Refrigerating perishable foods affects biochemical reactions by
A)improving
Q12: The activation energy of a chemical reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents