For the following reaction, the equilibrium constant 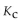 is 2.0 . If the concentration of both products is 0.10 M at equilibrium, what is the concentration of NOBr?
is 2.0 . If the concentration of both products is 0.10 M at equilibrium, what is the concentration of NOBr?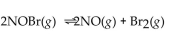
A) 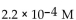
B) 2.2 M
C) 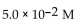
D) 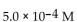
E) 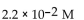
Correct Answer:
Verified
Q22: Q23: Q24: In the reaction of carbon dioxide with Q25: In an exothermic reaction, heat can be Q26: For the following reaction, the equilibrium constant Q28: When you open a bottle of a Q29: In the reaction of nitrogen gas with Q30: Carbon monoxide binds to hemoglobin 140 times Q31: Treatment of carbon monoxide poisoning can be Q32: In the following gas phase reaction, what![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents