For the following reaction, the equilibrium constant 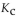 is 0.60 at a certain temperature. If the concentration of NO(g) and NOBr(g) are both 0.50 M ,at equilibrium, what is the concentration of
is 0.60 at a certain temperature. If the concentration of NO(g) and NOBr(g) are both 0.50 M ,at equilibrium, what is the concentration of 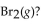
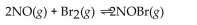
A) 0.60 M
B) 1.7 M
C) 2.8 M
D) 1.0 M
E) 0.36 M
Correct Answer:
Verified
Q32: In the following gas phase reaction, what
Q33: Q34: The physiological equilibrium system that keeps the Q35: For the following equilibrium reaction, which cause Q36: Q38: Iron metal reacts with oxygen gas to Q39: For the following reaction, the equilibrium constant Q40: For the following reaction, the equilibrium constant Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()

